Sage Therapeutics (SAGE: 42.98): “A New Form of Medicine”
By:
Hannah Cehaic, AIM Student at Marquette University
Disclosure: This article was written by myself, and it expresses my own opinions. I am not receiving compensation for it, and I have no business relationship with any company whose stock is mentioned in this article.
Summary:
- SAGE Therapeutics (NYSE: SAGE) is a clinical stage biopharmaceutical company, which engages in the development and commercialization of novel medicines to treat life-altering central nervous system. The firm’s business segment is solely made up of Novel Medicines (100%).
- SAGE Therapeutics generated a revenue CAGR of 13.03% from 2016 to November of 2021.
- SAGE is currently in phase 3 of FDA testing for a new antidepressant called ‘Zuranolone’ which has indications in major depressive disorder, postpartum depression, treatment resistant depression, generalized anxiety disorder, and bipolar depression. Zuranolone is being evaluated as a potential rapid-acting, durable, once-daily, two-week treatment.
- Barry E. Greene joined Sage as CEO in December of 2020, bringing more than 30 years of biopharmaceutical industry experience to this position. He previously joined the Sage Board of Directors in October of 2020.
Key Points:
SAGE is advancing a portfolio of clinical programs featuring internally discovered novel chemical entities with the potential to become differentiated products designed to improve brain health. SAGE is currently in phase 3 of FDA testing for a new antidepressant called ‘Zuranolone’. SAGE recently announced their plan to submit a New Drug Application (NDA) to the FDA for Zuranolone in the second half of 2022. The initial submission package will seek approval of Zuranolone for postpartum depression is anticipated in the first half of 2023.
Greene is in the process also of the creation of a new compound in the neurology indication called SAGE-324. This indication would help with essential tremors and Parkinson’s disease. Although there is only one Parkinson drug in the market, it does not compare with what SAGE Therapeutics as the company wants to provide an indication for essential tremors as well.
Research and development expenses were $66.2 million, including $13.5 million of non-cash stock-based compensation expense, in the second quarter of 2021 compared to $73.3 million, including $10.1 million of non-cash stock-based compensation expense, for the same period in 2020, a decrease of $7.1 million. The amount for the second quarter of 2021 reflects an increase in expenses of $13.0 million and a reduction in expenses of $20.1 million due to reimbursement from Biogen pursuant to the Sage/Biogen Collaboration and License Agreement. While net revenue from sales of ZULRESSO, their first ever patent antidepressant, was $1.6 million in the second quarter of 2021 compared to $1.1 million in the same period of 2020.
What has the stock done
lately?
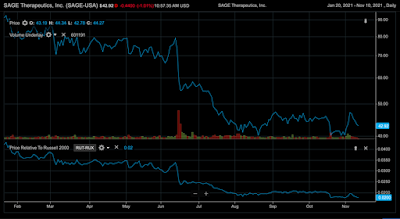
SAGE Therapeutics took a huge hit March 1st, right at the height of the pandemic and the market shut down. SAGE Therapeutics has been fluctuating these past few months because of the constant trials and testing. However, from October to November, there has been an 11.47% growth. Due to SAGE Therapeutics being a newer established company, they give out no dividends as of now.
Past Year Performance:
Over the past 12 months, SAGE has decreased 1.87% compared with the Russell 2000. Additionally, SAGE has seen a -44.60% change over the year due to the extra spending on the new drug patents and collaboration with the FDA. The amount for the second quarter of 2021 reflects an increase in expenses of $13.0 million and a reduction in expenses of $20.1 million due to reimbursement from Biogen pursuant to the Sage/Biogen Collaboration and License Agreement for the new antidepressant. The reasons for the increase in expenses were clinical pharmacology studies that began in 2021 and non-cash stock-based compensation expense.
My Take:
Each year, mental health is taken more
seriously. Even though the pandemic, the harsh trails of the FDA, and creating
a license for this new antidepressant has decreased the revenue, there is a lot
of potential. This is so because the new Zuranolone is the first antidepressant with an indication for
postpartum depression. If SAGE can get this approved by the FDA, SAGE would
create a moat for themselves in which no other company can compete with the new
antidepressant on the market. My recommendation would be to buy low and then
hold it until 2022/2023 when they can get through their third trail of testing
and finally market this new antidepressant.